We are UNIMED.
Unimed provides medical supplies, equipment, oxygen and clinical facilities management to cruise passenger, commercial and private marine clients worldwide. We comply with World Health Organization (WHO), United States Drug Enforcement Administration (DEA), Medicines and Healthcare products Regulatory Authority (MHRA), and Flags of Convenience requirements.
Global leaders with unparalleled professionalism.
Unimed employees undergo Good Distribution Practice (GDP) Awareness Training ensuring the proper handling, storage, and delivery of products. Employees also receive Counterfeit & Falsified Medicines Training and follow Defective Medicines Reporting processes. Unimed distribution centers are maintained with precise seasonal temperature mapping with daily temperature recordings to the tenth of a degree. These essential practices ensure product quality and integrity.
In compliance with universal data protection regulations.
Unimed complies with UK and EU General Data Protection Regulation (GDPR) legislation replacing the 1995 Data Protection Directive. The GDPR 2016/679 addresses the export of personal data outside the EU. The GDPR gives control to citizens and residents over their personal data and simplifies the international business regulatory environment .

ISO 9001:2015 QUALITY

ISO 14001:2015 ENVIRONMENTAL
We're Global
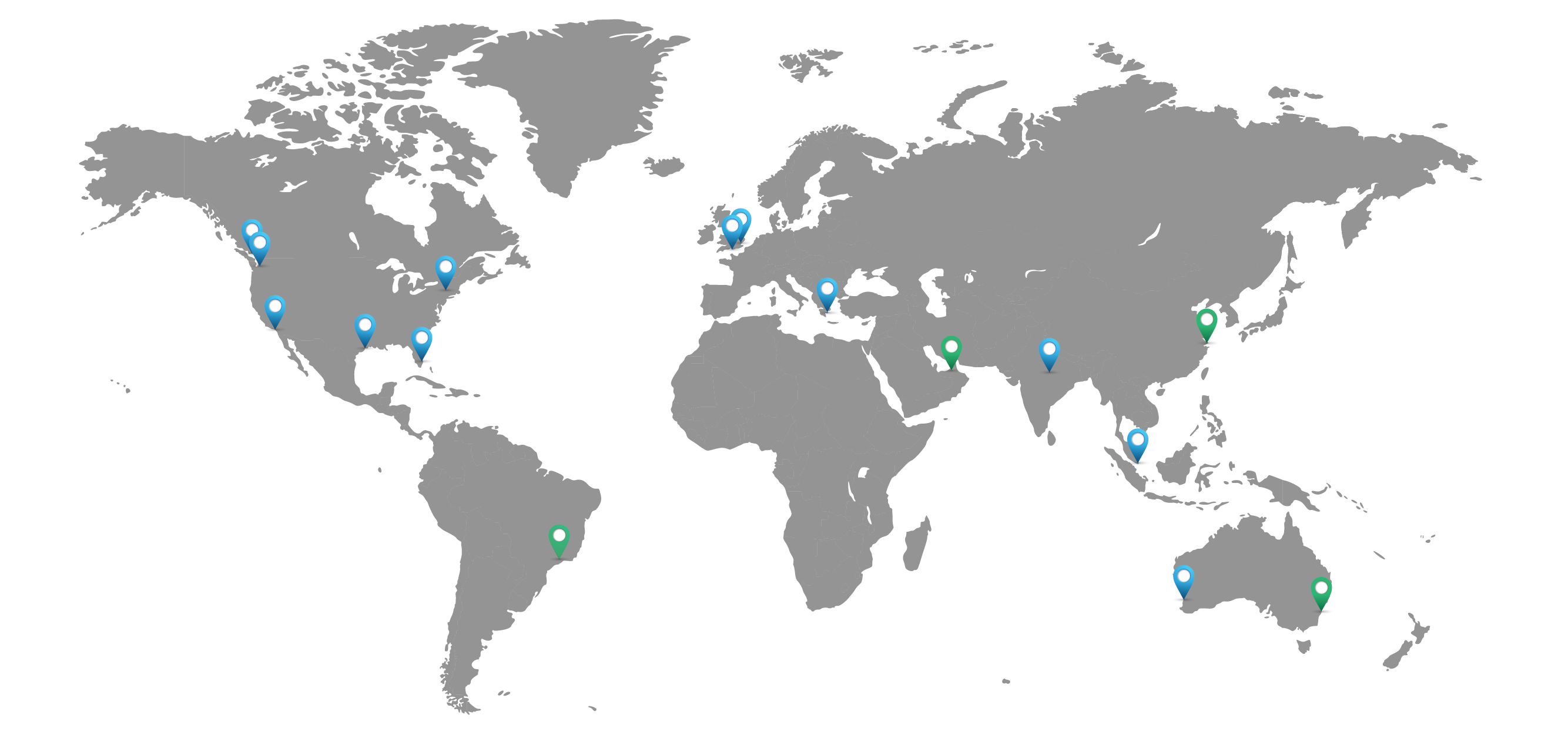
Getting You What You Need, When and Where You Need It
North America
Houston, TXLos Angeles, CA
Miami, FL
South Plainfield, NJ
Seattle, WA
Vancouver, CA
Fort Lauderdale, FL
Europe
Athens, GRLondon, UK
Southampton, UK
Asia - Pacific
New Delhi, INSingapore, SG
Perth, AU
Supply Branch Offices
Dubai, UAESantos, BR
Shanghai, CN
Sydney, AU